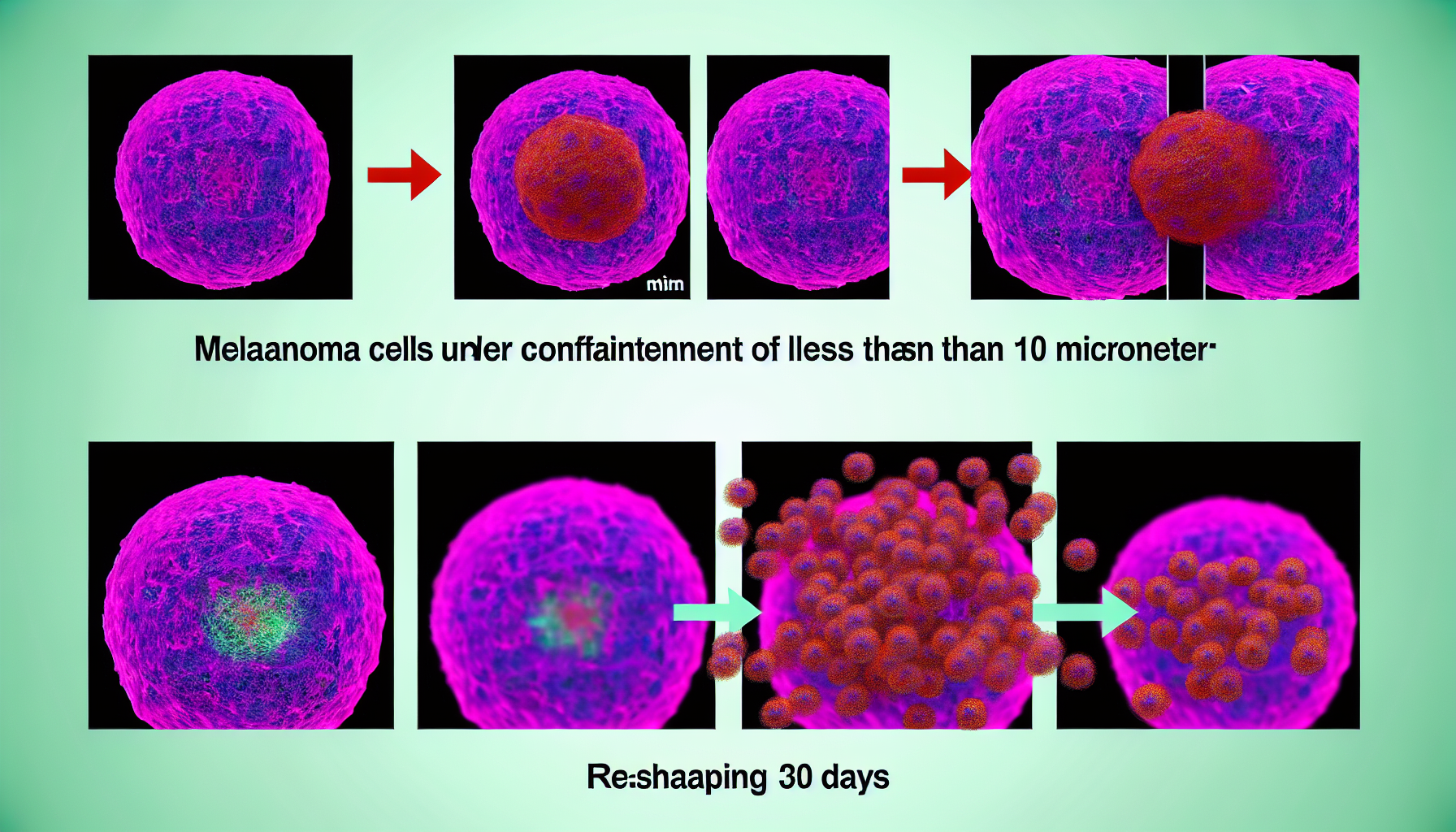
Mechanical forces are rewriting the melanoma playbook. New evidence shows that melanoma confinement—forcing cancer cells through channels narrower than 10 micrometres—rapidly reprograms their identity, making them behave more like stem cells that can survive stress, travel, and seed new tumors. Within minutes, cells squeezed through micro-sized spaces switch on stem-like programs; within weeks in animals, they form more metastases. Together, the findings recast metastasis as not only a genetic lottery but also a physical process shaped by the geometry of blood vessels and tissues [1].
Key Takeaways
– Shows squeezing melanoma cells through <10 μm channels induces stem-like proteins within 15 minutes, compared with 5–30 μm microchannel controls. - Reveals mice injected with 5–10 μm–squeezed melanoma cells formed significantly more lung, bone, and brain metastases after 30 days of monitoring. - Demonstrates 2025 Nature Communications data: melanoma confinement activates HMGB2-driven chromatin remodeling within minutes, producing acetylated-tubulin cages, reduced proliferation, and increased drug resistance. - Indicates tumor microtopology matters: 20 μm-width shapes maximized CD271 stem-like phenotype with ~94% correlation, linking integrin α5β1 and ERK signaling to angiogenesis. - Suggests transit through ~5–10 μm capillaries reprograms circulating cells, enabling prognostic blood assays and mechanics-targeted therapies to reduce metastasis risk.
How melanoma confinement under 10 μm reprograms cells
In a September 2025 report, researchers reproduced the tightest bottlenecks that circulating tumor cells encounter by pushing human melanoma cells through polydimethylsiloxane microchannels ranging from 5 to 30 micrometres. A clear threshold emerged: when channel widths dipped below 10 μm, cells began expressing stem-like proteins within just 15 minutes. The rapid onset underscores a mechanosensitive switch rather than a slow transcriptional drift [1].
The speed and size dependence matter. A 10 μm cutoff aligns with the smallest arterioles and capillary constrictions in vivo, indicating that ordinary vascular passage can trigger state changes. Because the induction occurs within minutes, the data suggest a fast-acting mechanotransduction cascade—likely cytoskeletal and chromatin-linked—rather than selection for preexisting rare subclones.
Independent coverage of the same Nature Communications work emphasizes that deformation itself is the signal: microchannel transit under 10 μm was sufficient to push cells toward a more tumorigenic, stem-like state, with the deformation visible within 15 minutes. The summary also highlights the translational idea that modulating these physical cues could reduce metastasis risk and guide therapy design [3].
From microchannels to metastases: what 30 days revealed
In vivo tests strengthened the case that the physical squeeze is not just a lab curiosity. When mice were injected with melanoma cells pre-squeezed through 5–10 μm channels, they developed significantly more metastases across lungs, bone, and brain after 30 days than animals receiving unsqueezed cells. The timeline—one month—is clinically relevant for monitoring metastatic burden in preclinical models and connects the 15-minute reprogramming window to tangible disease spread [4].
Importantly, the researchers interpret these results as evidence that circulation-driven reprogramming during capillary transit can create metastasis-competent cells on the fly. That reframes standard narratives focused solely on rare metastasis-initiating subpopulations. If the act of squeezing can transiently grant stem-like traits, the number of cells capable of seeding tumors could expand dramatically during a single pass through the microvasculature.
The implication is quantitative: every time melanoma cells traverse constrictions of ~5–10 μm, the odds of a metastasis-competent state may rise, even if only for a limited window. That suggests intervention points around vascular mechanics, cytoskeletal responses, or chromatin states, and it motivates better counting of how often and how intensely circulating cells are mechanically confined in patients.
The mechanics of plasticity: HMGB2, chromatin and tubulin
The Nature Communications study ties this behavior to a definable molecular axis. Under mechanical confinement, melanoma cells upregulated HMGB2, a chromatin-binding protein associated with DNA architecture, and engaged broad chromatin remodeling. Confinement activated neuronal-like invasion programs, formed acetylated-tubulin “cages” that stabilize deformed nuclei, reduced proliferation, and increased drug resistance—classic hallmarks of an invasive, therapy-tolerant phenotype. Disrupting HMGB2 shifted the balance between invasive and proliferative states and altered therapy responses in model systems, delineating a mechanistic bridge between squeeze, state, and survival [2].
Mechanistically, this is a playbook for phenotypic plasticity. The cytoskeleton absorbs and transmits stress; the nucleus reshapes; chromatin opens or tightens; and transcriptional programs that favor survival in harsh environments switch on. The result is a transient but consequential stem-like state that may persist long enough for extravasation, niche colonization, and eventual outgrowth once proliferation resumes.
Tumor topology backs the case for melanoma confinement
The vascular squeeze story aligns with earlier evidence that geometry alone can bias melanoma cells toward stem-like behavior. In 2017, researchers showed that microconfinement and curvature on engineered substrates elevated the melanoma-initiating cell phenotype: cells arranged on 20 μm-width shapes displayed the strongest link to the stem-like marker CD271, with an approximate 0.94 correlation. The pathway implicated integrin α5β1 and ERK signaling and increased in vitro tube formation, connecting mechanical context, stemness markers, and pro-angiogenic behavior [5].
That 20 μm topological optimum complements the <10 μm microchannel threshold by framing a broader range of geometric cues—from surface curvature to through-channel restraint—that can flip melanoma cells into a tumor-initiating, pro-angiogenic state. Together, the datasets suggest that both the shapes cells adhere to and the spaces they are forced through can tune stem-like programs quantifiably.
Why melanoma confinement matters for therapy and diagnostics
Therapeutically, if sub-10 μm transit primes melanoma cells for metastasis within 15 minutes, targeting the mechano-epigenetic response could blunt dissemination without needing to eliminate every circulating cell. Strategies might include dampening the cytoskeletal changes that stabilize deformed nuclei, modulating chromatin accessibility linked to HMGB2, or altering vascular mechanics to reduce extreme constrictions. Even small reductions in the frequency or severity of confinement events could produce outsized effects on metastatic seeding probability.
On diagnostics, the idea that capillary transit itself reprograms cells points to prognostic blood assays that do more than count circulating tumor cells. Assays could measure deformation history, stem-like marker induction post-transit, or HMGB2-linked chromatin states. Sampling kinetics could also matter: because the shift appears within minutes and metastases accrue over 30 days in mice, time-resolved blood profiling around surgery, biopsy, or therapy cycles might capture peaks of confinement-induced plasticity.
Quantifying the thresholds and time windows of risk
The numbers in this research define testable thresholds. A geometric cutoff—less than 10 μm—triggers stem-like markers within roughly 15 minutes; a biological outcome—more metastases—emerges after 30 days in vivo. Future studies can refine the dose-response: for example, how 5 μm compares with 7 μm or 9 μm constrictions; how many squeezes are needed; and how long the stem-like state lasts before cells revert.
Quantitatively mapping those curves would enable risk models: given a distribution of vessel diameters and transit frequencies, one could estimate the proportion of circulating melanoma cells that become metastasis-competent on any given day. Those models could then be linked to drug timing, sequence, or dosing strategies aimed at the minutes-to-hours window when confinement-induced programs are most active.
Integrating melanoma confinement into clinical workflows
For clinicians, the most pragmatic near-term steps are measurement and monitoring. Imaging-derived estimates of microvascular diameters in primary and metastatic sites could be combined with circulating tumor cell assays that test for stem-like markers shortly after collection. If a patient’s circulation imposes frequent sub-10 μm transits, the metastasis risk profile might be higher—and more responsive to interventions targeting cytoskeletal dynamics or chromatin remodeling.
Because the plasticity is reversible and context-dependent, combining confinement-aware strategies with standard-of-care therapies may be key. For instance, if confinement temporarily suppresses proliferation and raises drug tolerance, scheduling cytotoxic agents when cells are more proliferative—and pairing them with agents that block confinement-induced programs—could improve response rates. Prospective trials will be needed to quantify benefits.
What researchers still need to measure
Two gaps stand out. First, persistence: we know stem-like proteins rise within 15 minutes of sub-10 μm confinement, but the decay kinetics in vivo remain to be mapped. Second, heterogeneity: different melanoma genotypes and microenvironments may shift the exact threshold or magnitude of the response, complicating universal cutoffs. Carefully stratified datasets—with standardized channel sizes, transit counts, and longitudinal readouts—will clarify variance and confidence intervals.
Finally, organ specificity deserves attention. The mouse data show increases across lung, bone, and brain after 30 days, but each organ’s microvascular architecture and immune milieu differ. Quantitative comparisons of metastatic burden per organ versus measured confinement exposure will help identify where—and when—mechanics most powerfully seed disease.
Sources: [1] MedicalXpress – Squeezing through tiny blood vessels may trigger melanoma cells to spread: https://medicalxpress.com/news/2025-09-tiny-blood-vessels-trigger-melanoma.html [2] Nature Communications (via PubMed) – Mechanical confinement governs phenotypic plasticity in melanoma: https://pubmed.ncbi.nlm.nih.gov/40866703/ [3] TechnologyNetworks – Mechanical stress from blood vessels aids tumor metastasis: www.technologynetworks.com/cell-science/news/mechanical-stress-from-blood-vessels-aids-tumor-metastasis-404418″ target=”_blank” rel=”nofollow noopener noreferrer”>https://www.technologynetworks.com/cell-science/news/mechanical-stress-from-blood-vessels-aids-tumor-metastasis-404418 [4] BioCompare – Squeezing Cancer Cells Offers New Insight into Metastasis: www.biocompare.com/Life-Science-News/621211-Squeezing-Cancer-Cells-Offers-New-Insight-into-Metastasis/” target=”_blank” rel=”nofollow noopener noreferrer”>https://www.biocompare.com/Life-Science-News/621211-Squeezing-Cancer-Cells-Offers-New-Insight-into-Metastasis/ [5] Science Advances – Melanoma topology reveals a stem-like phenotype that promotes angiogenesis: www.science.org/doi/full/10.1126/sciadv.1701350″ target=”_blank” rel=”nofollow noopener noreferrer”>https://www.science.org/doi/full/10.1126/sciadv.1701350 TARGET_KEYWORDS: [melanoma confinement, <10 μm channels, 5–10 μm microchannels, 5–30 μm range, 15-minute induction, 30-day metastasis, HMGB2 upregulation, chromatin remodeling, acetylated tubulin cages, CD271 correlation 0.94, 20 μm shapes, integrin α5β1, ERK signaling, stem-like phenotype, drug resistance increase, phenotype switching, neuronal invasion programs, zebrafish models 2025, PDMS microchannels, metastasis risk reduction] FOCUS_KEYWORDS: [melanoma confinement, sub-10 μm confinement, 15-minute stem-like shift, 30-day metastasis burden, HMGB2 mechanotransduction, CD271 94% correlation, 20 μm topology] SEMANTIC_KEYWORDS: [phenotypic plasticity, mechanotransduction, microvascular constriction, capillary transit, tumorigenicity, metastasis seeding, invasive-proliferative trade-off, therapy resistance, angiogenesis, cytoskeletal deformation, nuclear mechanics, prognostic blood assay] LONG_TAIL_KEYWORDS: [melanoma confinement under 10 μm effects, 15-minute stem-like induction melanoma, 30-day metastasis increase mouse models, HMGB2 chromatin remodeling melanoma, acetylated tubulin cages cancer squeeze, 20 μm topology CD271 correlation 0.94, integrin α5β1 ERK melanoma stemness, PDMS microchannels 5–30 μm melanoma, mechanics-targeted therapies metastasis] FEATURED_SNIPPET: Melanoma confinement—squeezing cells through channels under 10 μm—triggers a stem-like shift within 15 minutes and increases metastases after 30 days in mice. The 2025 study links this rapid plasticity to HMGB2-driven chromatin remodeling and acetylated-tubulin “cages,” while earlier topology work found a ~94% CD271 correlation on 20 μm shapes. Together, the data show mechanical stress can reprogram melanoma and elevate metastasis risk.
Image generated by DALL-E 3





Leave a Reply