H5N1 pasteurization is delivering on its purpose: fully inactivating infectious bird flu virus in milk at standard commercial settings, even when tests still pick up non-infectious RNA fragments. Across multiple datasets, laboratory and retail evidence converge on the same conclusion—milk processed via high-temperature, short-time (HTST) pasteurization at 72°C for 15 seconds is safe. At the same time, surveillance continues to detect viral genetic material in a minority of retail products, underscoring the difference between detection of fragments and the presence of live, infectious virus.
Key Takeaways
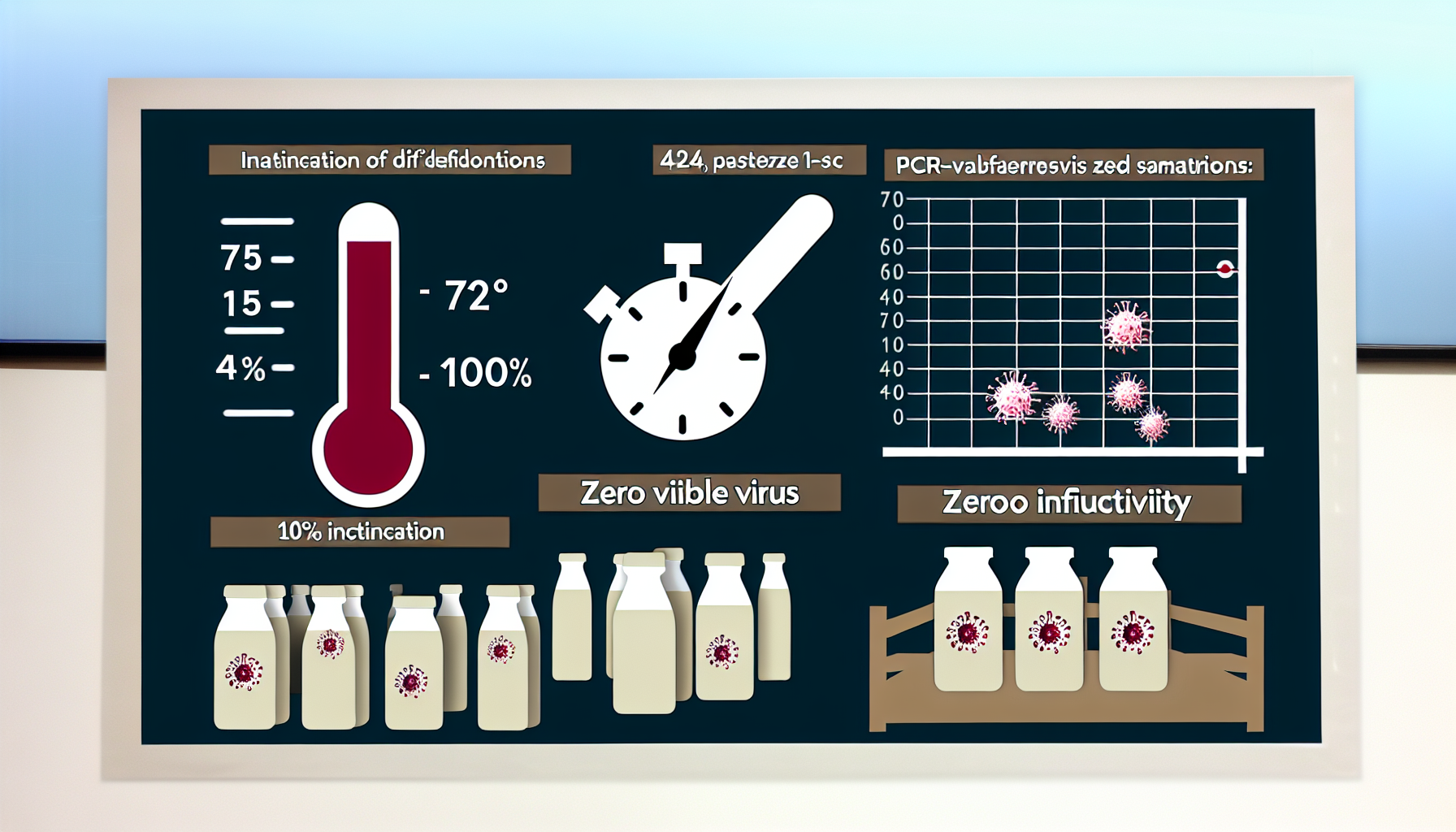
– Shows HTST at 72°C for 15 seconds inactivated 100% of infectious H5N1 in milk; RNA sometimes detectable via PCR, not live virus. – Reveals 464 pasteurized dairy products tested nationwide contained zero viable H5N1, affirming commercial pasteurization safety across the U.S. supply chain. – Demonstrates heating raw milk at 54°C for 15 minutes or 60°C for 10 seconds was sufficient to inactivate H5N1 in trials. – Indicates about 20% of 297 retail milk samples from 17 states carried viral RNA, yet no infectious virus was cultured from any. – Suggests overall public health risk remains low, with WHO advising consumption of pasteurized milk and continued surveillance during dairy outbreaks.
What the science says about H5N1 pasteurization
In a March 2024 New England Journal of Medicine letter, researchers showed HTST pasteurization (72°C for 15 seconds) completely inactivated infectious H5N1 in milk, even though PCR occasionally detected viral RNA fragments and retail surveys found signals without any culturable virus [1].
An FDA update on September 30, 2024 reported that 464 pasteurized dairy products contained no viable H5N1, and laboratory work confirmed inactivation by heating raw milk at 54°C for 15 minutes or 60°C for 10 seconds, reinforcing confidence in standard 161°F (72°C) for 15 seconds HTST processes [2].
Complementing this, a Journal of Virology study summarized by the American Society for Microbiology on July 3, 2024 tested 297 retail milk products across 17 states: about 20% were PCR-positive for genetic material, yet none yielded infectious virus in culture, supporting “milk is safe” under pasteurization [3].
Why H5N1 pasteurization can leave detectable RNA
PCR is exquisitely sensitive to genetic fragments. When milk containing virus passes through a validated pasteurization profile, the thermal hit denatures proteins and disrupts the viral envelope, ending infectivity. However, segments of RNA—and sometimes protein fragments—can persist long enough to be picked up by molecular assays that do not differentiate between intact, infectious virions and harmless debris.
This is why laboratory culture remains the gold standard for determining infectivity. A sample can be PCR-positive yet culture-negative if the virus has been inactivated. In the datasets reported to date, culture methods have consistently failed to grow live H5N1 from pasteurized retail milk that registered molecular signals, aligning with thermal inactivation kinetics.
Another factor is how milk moves through the supply chain. Viral material shed upstream on farms can enter raw milk before pasteurization. Pasteurization neutralizes infectivity, but post-process testing may still detect remnants at low concentrations. The key epidemiological question—can it infect?—is answered by the negative culture results and the absence of viable virus in broad retail testing.
The public health picture and consumer guidance
For consumers, two data-driven points stand out. First, HTST at 72°C for 15 seconds achieves total inactivation in controlled experiments. Second, large retail surveys report zero viable virus despite molecular detections. Together, these reinforce what food safety systems are built to deliver: multiple layers—sanitation on farm, validated pasteurization in plants, and ongoing monitoring—reducing risk to a low level.
The World Health Organization said on April 26, 2024 that the overall public health risk from H5N1 remained low, urging vigilance and recommending consumption of only pasteurized milk during outbreaks, noting reports of particles in roughly 20% of samples but no viable virus [5].
Practical advice remains straightforward. Choose pasteurized dairy; avoid raw milk, which lacks the validated heat step. For vulnerable groups—infants, older adults, pregnant people, and those who are immunocompromised—the pasteurized-only rule is especially important. If supply disruptions occur during animal outbreaks, follow local health advisories and brand-level recalls, which will reflect real-time surveillance.
Methodology, limits, and what to watch next
It is useful to unpack the designs behind the headline numbers. The Journal of Virology retail study took a broad snapshot: 297 products from 17 states, a diverse cross-section that can reveal how widespread RNA signals are in finished goods. The FDA’s 464-product analysis adds national scope and regulatory sampling rigor, with a clear outcome: no viable virus detected in pasteurized retail items.
Thermal challenge experiments make the picture more quantitative. Beyond the standard HTST setting, lower-temperature, longer-time and short-burst heating trials (e.g., 54°C for 15 minutes; 60°C for 10 seconds) also rendered virus non-infectious in controlled settings. Those results help triangulate inactivation thresholds and provide redundancy should processors need to validate alternative time–temperature profiles in specific equipment configurations.
There are still scientific threads to follow. What are the exact log reductions at incremental temperatures? How do fat content and solids-not-fat affect thermal transfer and envelope disruption? What limits of detection are practical for differentiating low-level RNA fragments from any rare residual infectivity in edge cases? Regulators and academics are likely to refine these parameters as more controlled datasets accumulate.
Earlier, STAT reported on April 23, 2024 that while PCR-positive signals had been detected in pasteurized milk, multiple laboratories, including at the University of Wisconsin, found pasteurization likely inactivates H5N1, with experts awaiting further USDA validation studies for regulators [4].
Turning H5N1 pasteurization data into action for processors
For dairy processors, the evidence-base translates into actionable verification. First, confirm heat exchangers consistently reach and hold 72°C for 15 seconds (or the validated equivalent) across all flow rates and viscosities. Second, calibrate and document recording thermometers and flow-diversion valves; minor deviations can erode safety margins, especially at line start-up or during product switches.
Third, strengthen pre-pasteurization hygiene to minimize upstream viral material entering raw milk silos. This includes enhanced equipment sanitation, targeted environmental swabbing on raw-side surfaces, and supplier audits focusing on on-farm biosecurity. Reducing the incoming bioburden decreases the likelihood of downstream molecular positives that, while non-infectious, can trigger costly investigations.
Fourth, maintain a layered verification plan. Combine continuous temperature recording with periodic microbial culture-based checks and, where appropriate, molecular screening calibrated to avoid overinterpreting fragments. Align your hazard analysis critical control point (HACCP) plan with current regulatory guidance and be prepared to adjust testing frequencies dynamically during outbreaks affecting dairy herds.
What consumers should know about labels and terms
Pasteurized milk in the United States overwhelmingly uses HTST processing—high temperature for a short time—to balance safety and quality. Some products may be labeled “ultra-pasteurized” or use alternative validated profiles, but the principle is the same: a defined time–temperature kill step verified by instrumentation and logged for compliance.
If you see headlines about “viral particles” detected in milk, remember the distinction. Molecular assays are built to detect fragments, increasing sensitivity for surveillance. Culture results and retail testing tell you whether anything infectious remains. In the current evidence, the answer is no in pasteurized products sampled across states and brands, even when a fraction of them were PCR-positive.
Why H5N1 pasteurization matters for outbreak resilience
The dairy supply chain is large, fast-moving, and geographically distributed. Validated pasteurization provides a robust, measurable firewall that remains reliable even when upstream surveillance finds viral material in raw milk during animal outbreaks. That firewall is quantifiable—72°C for 15 seconds—and it has been cross-checked in both laboratory and marketplace sampling.
Consumer confidence hinges on two capabilities: eliminating infectivity and proving it transparently. The convergence of culture-negative retail testing, challenge trials at multiple temperatures, and consistent messages from regulators and global health agencies all point to the same conclusion. Pasteurized milk remains a safe choice while surveillance and biosecurity continue to improve on farms.
The bottom line on H5N1 pasteurization
H5N1 pasteurization delivers complete inactivation at standard HTST settings and shows a reassuring safety margin at additional time–temperature combinations. Detectable RNA is not the same as viable virus; culture and infectivity data carry the weight in risk assessment. With 464 pasteurized products culture-negative for H5N1 and roughly one in five retail samples showing only non-infectious fragments, the data are internally consistent: pasteurized milk remains a low-risk, high-confidence option for consumers.
Sources:
[1] New England Journal of Medicine – Inactivation of Avian Influenza A(H5N1) Virus in Raw Milk at 63°C and 72°C: www.nejm.org/doi/full/10.1056/NEJMc2405488″ target=”_blank” rel=”nofollow noopener noreferrer”>https://www.nejm.org/doi/full/10.1056/NEJMc2405488
[2] U.S. Food and Drug Administration (FDA) – Investigation of Avian Influenza A (H5N1) Virus in Dairy Cattle: www.fda.gov/food/alerts-advisories-safety-information/investigation-avian-influenza-h5n1-virus-dairy-cattle” target=”_blank” rel=”nofollow noopener noreferrer”>https://www.fda.gov/food/alerts-advisories-safety-information/investigation-avian-influenza-h5n1-virus-dairy-cattle [3] American Society for Microbiology – Pasteurization Inactivates Highly Infectious Avian Flu in Milk: https://asm.org/press-releases/2024/july/pasteurization-inactivates-highly-infectious-avian
[4] STAT – H5N1 bird flu particles found in pasteurized milk; FDA backs safety: www.statnews.com/2024/04/23/h5n1-bird-flu-virus-particles-in-pasteurized-milk-fda/” target=”_blank” rel=”nofollow noopener noreferrer”>https://www.statnews.com/2024/04/23/h5n1-bird-flu-virus-particles-in-pasteurized-milk-fda/ [5] Reuters – WHO says bird flu risk currently low, asks countries to remain vigilant: www.reuters.com/world/who-says-public-health-risk-posed-by-bird-flu-virus-is-low-2024-04-26/” target=”_blank” rel=”nofollow noopener noreferrer”>https://www.reuters.com/world/who-says-public-health-risk-posed-by-bird-flu-virus-is-low-2024-04-26/
Image generated by DALL-E 3






Leave a Reply