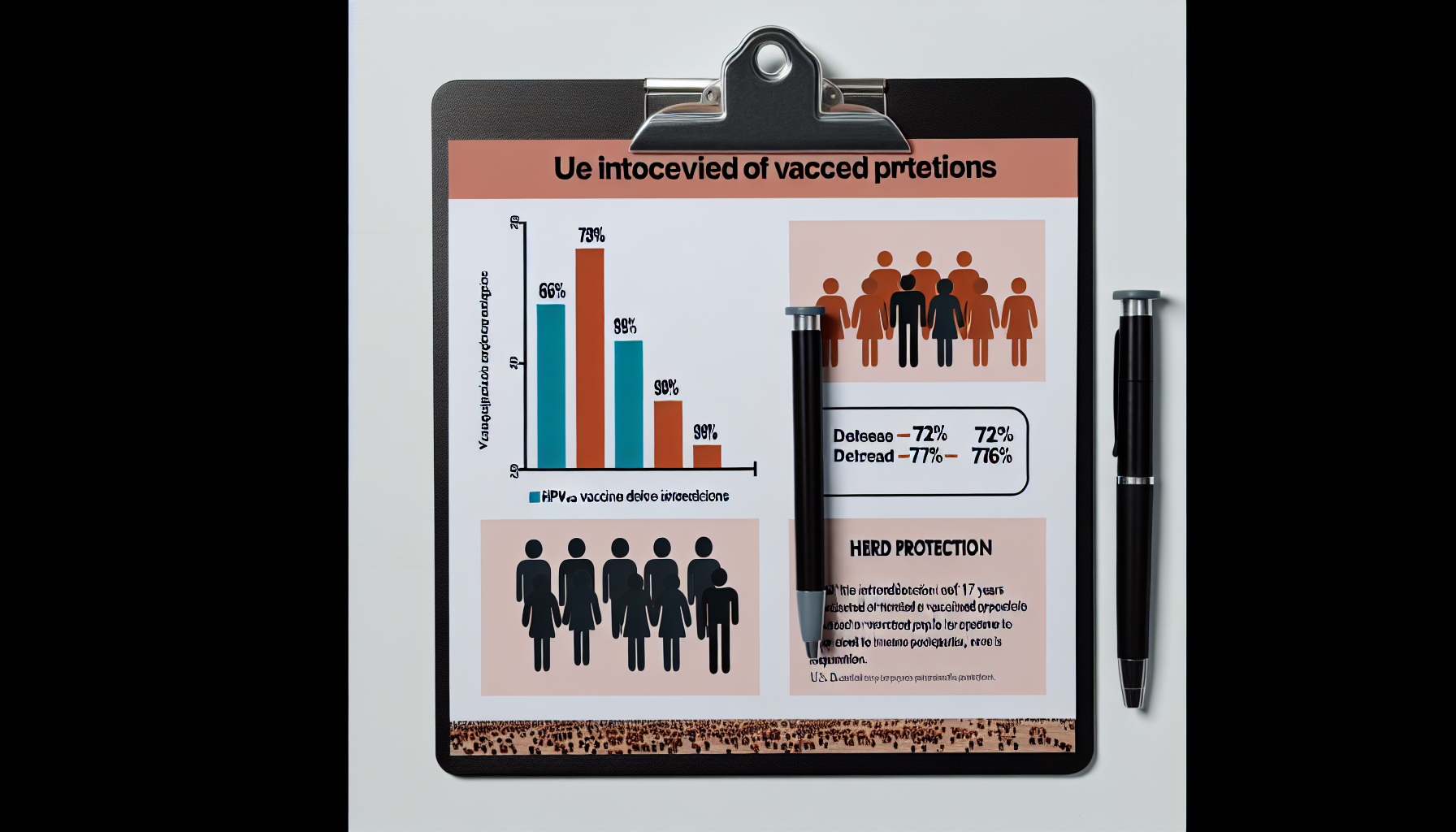
Seventeen years after its U.S. debut, the HPV vaccine is delivering substantial population-level protection. New evidence shows vaccine-type HPV infections fell by 76% to 98% among vaccinated women, depending on formulation, and declined sharply even among unvaccinated peers, signaling potent herd protection as coverage expanded from 2006 to 2023 [1].
Key Takeaways
– shows vaccine‑type HPV positivity dropped 98.4% (2‑valent), 94.2% (4‑valent), and 75.7% (9‑valent) among vaccinated women over 17 years. – reveals herd protection: unvaccinated women saw 71.6% and 75.8% declines in infections with 2‑ and 4‑valent vaccine types circulating. – demonstrates national surveillance gains: four‑type HPV infections fell up to 88% in girls 14–19 within 12 years of rollout. – indicates precancerous cervical lesions plunged about 80% among screened women aged 20–24 from 2008 to 2022, tracking broad vaccination uptake. – suggests early dosing matters: starting the HPV vaccine before age 17 correlates with an 88% lower risk of invasive cervical cancer.
How the HPV vaccine reshaped infection rates
A new cross-sectional study in JAMA Pediatrics analyzed 2,335 females aged 13–26 between 2006 and 2023, tracking vaccine-type HPV positivity as U.S. vaccination programs matured [1]. Among vaccinated participants, vaccine-type infections plummeted 98.4% for the 2‑valent, 94.2% for the 4‑valent, and 75.7% for the 9‑valent formulations, underscoring strong real-world effectiveness across product generations [1].
The same analysis found dramatic indirect effects. Among unvaccinated women, infections with types targeted by the 2‑ and 4‑valent vaccines fell 71.6% and 75.8%, respectively, consistent with robust herd protection as community coverage increased [1]. By the end of the study period, vaccination uptake in the cohort had risen to 82.1%, a key driver of these population-level declines [4].
These results reflect nearly two decades of vaccine introduction, scale-up, and product evolution from 2‑ to 9‑valent formulations. The data capture real-world dynamics as cohorts exposed to routine recommendations aged into adolescence and young adulthood, where HPV exposure risk rises and vaccine protection is most consequential [1].
HPV vaccine herd protection extends to unvaccinated women
The declines among unvaccinated women—71.6% and 75.8% for 2‑ and 4‑valent vaccine-type infections—highlight a central epidemiologic benefit: herd protection [1]. As more individuals are immunized, community transmission of vaccine-targeted HPV types diminishes, indirectly shielding those who are not vaccinated or not fully vaccinated [1].
Investigators interpret these findings as powerful, real-world evidence that high coverage does more than protect individuals—it lowers the overall force of infection in the population [1]. Public health leaders involved with the study have emphasized that such community-wide gains are critical for cancer prevention strategies and support continued expansion of access and uptake [4].
The herd effect is not a substitute for personal protection. Rather, it amplifies benefits when vaccination programs achieve broad reach, particularly before sexual debut, aligning with routine immunization at ages 11–12 and catch-up recommendations [2]. The JAMA Pediatrics data quantify that amplification over 17 years of program experience [1].
U.S. surveillance trends validate HPV vaccine impact
National surveillance compiled by the CDC corroborates the study’s trajectory. Within four years of rollout, vaccine-type HPV infections among females aged 14–19 fell 56%, and within 12 years, infections with the four type-specific targets dropped by as much as 88% in that age group [2]. The CDC also reports marked reductions in genital warts and cervical precancers, indicating benefits that extend beyond infection prevalence into disease outcomes [2].
Clinical outcomes are moving in parallel. A CDC analysis reported by AP News found roughly an 80% decrease in precancerous cervical lesions among screened women aged 20–24 from 2008 to 2022, a period spanning the maturation of routine vaccination for girls (2006) and boys (2011) [3]. Such declines strongly suggest the HPV vaccine is preventing disease that would otherwise progress without immunization [3].
These surveillance and clinical trends reduce uncertainty about translation from virologic endpoints to health outcomes. Declines in vaccine-type infections, genital warts, and high-grade lesions cohere with the expected impact of a high-coverage vaccination program on HPV-related disease [2].
Early HPV vaccine dosing is tied to cancer prevention
A nationwide study published in the New England Journal of Medicine provides direct evidence on invasive cancer. Initiating the HPV vaccine before age 17 was associated with an 88% lower risk of invasive cervical cancer compared with never being vaccinated, with a 95% confidence interval of 66% to 100% [5]. The magnitude of this association supports early vaccination as a cornerstone of cancer prevention policy [5].
This aligns with CDC recommendations to start routine HPV vaccination at ages 11–12, capitalizing on immune responsiveness and ensuring protection is in place before exposure risk rises [2]. Together, the cancer-risk evidence and surveillance trends underscore that timing matters as much as coverage for maximizing public health gains [5].
What the numbers mean for HPV vaccine policy and access
The JAMA Pediatrics results arrive as programs strive to close coverage gaps and address inequities. Investigators and health-system leaders involved in the study have urged expanding global access, citing disparities that could blunt herd protection and delay progress toward eliminating cervical cancer as a public health problem [4]. The 82.1% uptake seen in the cohort demonstrates what is possible, but it is not universal [4].
Policy priorities that follow from the data include sustaining school-based and clinical reminder programs, removing cost and logistical barriers, and maintaining strong provider recommendations at the routine ages of 11–12 [2]. Because herd protection rises with coverage, each incremental gain can reduce transmission pressure and extend benefits to communities with lower uptake [1].
It is also essential to maintain cervical cancer screening, particularly for those who are unvaccinated or under-vaccinated, as vaccination does not retroactively treat prior infections and does not cover every oncogenic HPV type [2]. Surveillance of vaccine-type prevalence, precancer, and cancer incidence should continue to monitor long-term impact as vaccinated cohorts age [2].
Methodology and interpretation in context
The JAMA Pediatrics study’s cross-sectional design captured snapshots from 2006 to 2023, enabling comparisons across vaccine eras and coverage levels in 2,335 females aged 13–26 [1]. While cross-sectional data cannot establish individual causality, the scale of declines across vaccinated and unvaccinated groups, combined with independent national surveillance, strengthens causal inference at the population level [1].
Notably, the study reports vaccine-type positivity declines tightly aligned with the antigenic targets of the 2‑, 4‑, and 9‑valent vaccines, a biologically coherent pattern difficult to attribute to confounders [1]. The convergence of virologic, precancer, and cancer-risk evidence across datasets and designs supports the conclusion that vaccination is the principal driver of the observed reductions [2][5].
The bottom line on the HPV vaccine’s population impact
Across 17 years, the HPV vaccine has slashed vaccine-type infections by up to 98% among vaccinated women and markedly reduced infections among unvaccinated peers via herd protection [1]. U.S. surveillance shows parallel declines in vaccine-type prevalence, genital warts, and precancerous cervical lesions, while early vaccination is associated with a steep drop in invasive cervical cancer risk [2][3][5]. These converging data illustrate a durable, expanding shield against HPV-driven disease when coverage is high and doses are delivered early [1].
Sources:
[1] JAMA Pediatrics – Population‑Level Effectiveness and Herd Protection 17 Years After HPV Vaccine Introduction: https://jamanetwork.com/journals/jamapediatrics/article-abstract/2839024
[2] Centers for Disease Control and Prevention (CDC) – HPV Vaccine Safety and Effectiveness Data: www.cdc.gov/hpv/hcp/vaccination-considerations/safety-and-effectiveness-data.html” target=”_blank” rel=”nofollow noopener noreferrer”>https://www.cdc.gov/hpv/hcp/vaccination-considerations/safety-and-effectiveness-data.html [3] AP News – CDC report adds to evidence that HPV vaccine is preventing cervical cancer in US women: https://apnews.com/article/32cdcf325d12cbc6610f01430d4ee82b
[4] Montefiore Einstein / Cincinnati Children’s – Study Shows HPV Vaccine Protects Vaccinated—and Unvaccinated—Women: https://montefioreeinstein.org/news/2025/09/29/study-shows-hpv-vaccine-protects-vaccinated-and-unvaccinated-women [5] New England Journal of Medicine (NEJM) – HPV Vaccination and the Risk of Invasive Cervical Cancer: www.nejm.org/doi/10.1056/NEJMoa1917338″ target=”_blank” rel=”nofollow noopener noreferrer”>https://www.nejm.org/doi/10.1056/NEJMoa1917338
Image generated by DALL-E 3





Leave a Reply